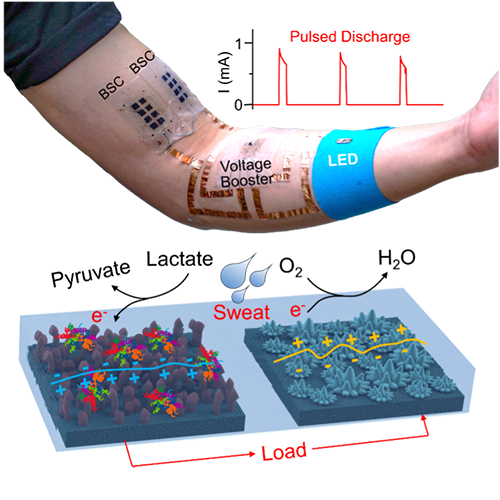
Self-charging biosupercapacitors (BSCs) that can store energy and be self-charged via chemical or solar energy conversion through bioreaction have recently attracted considerable attention. As human sweat also contains a high concentration of lactate biofuel, the harvesting and storage of the bioenergy in sweat holds the potential to provide the power for wearable electronics.
However, materials utilized in previous BSCs are either bulky, rigid, or fragile, and therefore cannot serve as suitable candidates for stretchable conformal wearable electronic devices. For a wearable BSC, the flexibility, stretchability, and skin conformity of the device are of considerable importance.
Prof. Joseph Wang’s group in UC San Diego demonstrates the first example of an all-printed dual-functional stretchable and wearable BSC, fabricated on top of low-elastic modulus and adhesive elastic films, to harvest and store energy from sweat while maintaining intimate contact with the human skin.
This wearable hybrid device, functioning as both a biofuel cell and a supercapacitor, is demonstrated to deliver high-power pulses and be rapidly self-recharged using enzymatic oxidation of lactate biofuel from human perspiration.
This work enabled material-level integration of both functionalities on the same set of electrodes, thus reducing the system complexity and minimizing the device footprint.
Discover Also