Watching cells breathe has always been difficult. The chemical reactions that keep our cells alive happen at scales too small and speeds too fast for most instruments to capture. Traditional molecular probes, the workhorses of cell biology, suffer from fundamental limitations: they bleach under light, they accumulate signals over time rather than reporting what is happening at any given moment, and they cannot provide the continuous, real-time monitoring that would reveal how metabolism truly unfolds.
Quantum sensing offers a different approach. At its heart lies an atomic-scale defect in diamond called the nitrogen-vacancy center, or NV center, a spot where a nitrogen atom sits next to a vacant lattice site in the carbon crystal. This tiny imperfection gives diamond remarkable quantum properties. When illuminated with green laser light, the NV center emits red fluorescence whose brightness depends on its local magnetic environment.
Magnetic resonance explains why this matters. The NV center has a characteristic resonance frequency of approximately 2.87 GHz. Paramagnetic molecules, those with unpaired electrons such as free radicals and certain metal ions, generate magnetic noise at frequencies that include this resonance. When such molecules approach the NV center, they accelerate the decay of its quantum state back to equilibrium. The time constant of this decay, known as T₁ or longitudinal relaxation time, becomes a direct readout of local paramagnetic concentration.
Since reactive oxygen species and redox-active metals play central roles in cellular metabolism, measuring T₁ inside living cells could provide a detailed view of metabolic activity. Previous work demonstrated that NV-based sensors could detect individual paramagnetic molecules in controlled laboratory settings and even sense intracellular radicals. Yet significant questions remained about whether such measurements would be stable, reversible, and specific enough to track real metabolic changes over meaningful timescales.
A study published in Advanced Functional Materials (« Subcellular Metabolic Tracking Using Fluorescent Nanodiamonds Relaxometry ») directly addresses these challenges. A research team at the University of Science and Technology of China has demonstrated the first simultaneous integration of spatial tracking with NV-based relaxometry inside living cells.
By functionalizing 40 nm fluorescent nanodiamonds to target specific organelles and developing methods to track individual particles as they move, the researchers created a platform for monitoring metabolic activity at the subcellular level. Importantly, cells retained greater than 95% viability over three days under their measurement conditions, validating the non-invasive nature of the approach.
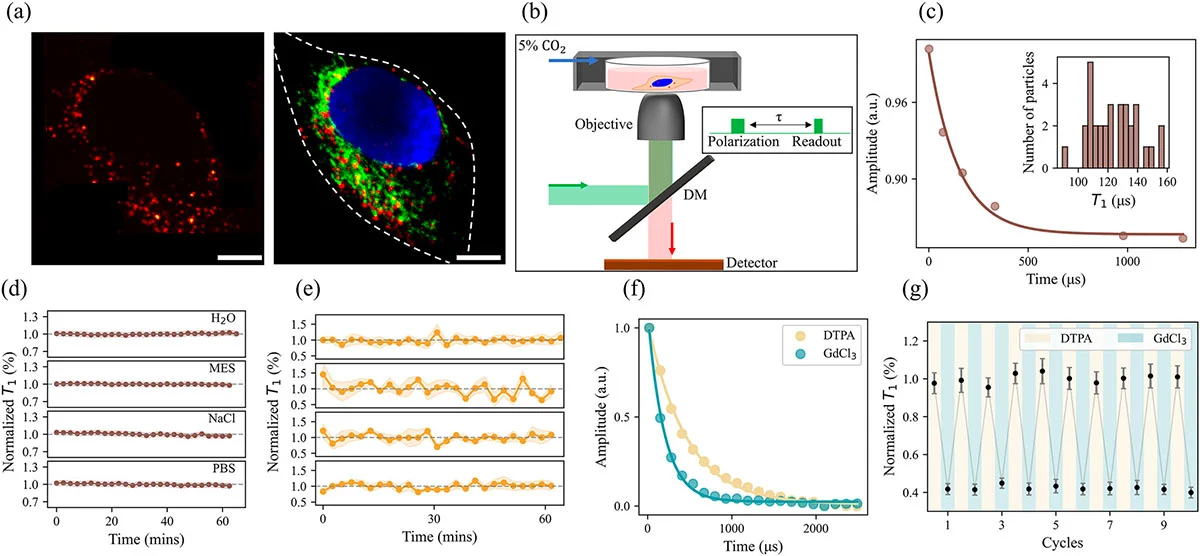
Fluorescent nanodiamonds inside a fixed human cervical cancer cell and validation of their stability and reversibility as quantum sensors. (a) Left panel: confocal microscopy image showing nanodiamonds (red) distributed throughout the cell. Right panel: wide-field image with merged channels displaying nanodiamonds (red), the nucleus stained with DAPI (blue), and mitochondria labeled with MitoTracker Green FM (green). White dotted lines indicate cell boundaries. Scale bars: 10 μm. (b) Schematic of the optical measurement system combining relaxometry with confocal and wide-field imaging. Inset shows the timing sequence for T₁ relaxation measurement: an initial laser pulse polarizes the electron spin, a variable dark time (τ) allows relaxation to occur, and a second laser pulse reads out the resulting fluorescence. (c) T₁ decay curve from a single nanodiamond within the cell shown in (a), yielding a relaxation time of 148.95 ± 38.94 μs. Inset: distribution of T₁ values measured from 32 individual nanodiamonds in the same cell, showing a mean of 124.11 ± 16.29 μs. (d) Stability of T₁ measurements over 60 minutes for nanodiamonds in water, MES buffer at pH 6.0, PBS, and 1 M NaCl solution, demonstrating robustness across different chemical environments. (e) Stability of T₁ measurements for four individual nanodiamonds tracked across three fixed cells over 60 minutes, confirming consistent performance in biological specimens. All T₁ values in (d) and (e) are normalized to the mean. (f) T₁ decay curves measured in the presence of the paramagnetic agent gadolinium chloride at 10 μM (blue) and after addition of the chelator DTPA at 100 μM (yellow), which removes gadolinium from solution. The faster decay with gadolinium present demonstrates the sensor’s responsiveness to paramagnetic species. (g) Reversibility test showing T₁ values over 10 alternating cycles of gadolinium chloride and DTPA exposure, confirming that the quantum sensors return fully to baseline after each cycle without signal degradation. Error bars in (d), (e), and (g) represent standard errors of the fits. (Image: Reproduced with permission from Wiley-VCH Verlag) (click on image to enlarge)
Systematic validation formed the foundation of this work. The researchers confirmed that T₁ values remained stable across various aqueous and saline environments, including water, MES buffer at pH 6.0, phosphate-buffered saline, and 1 M sodium chloride solution. This robustness to chemical conditions matters because the cellular interior fluctuates in pH and electrolyte composition.
Fixed cells provided the next test environment. These specimens eliminate metabolic variables while preserving cellular structures. Over measurement periods exceeding 1 h, T₁ values from individual nanodiamonds showed consistent stability with no significant monotonic trends, indicating the sensors remain unaffected by static cellular components.
Reversibility represents a critical requirement for any real-time sensor. Using gadolinium chloride, a paramagnetic compound, and its diamagnetic chelator DTPA, the researchers demonstrated that T₁ changes were fully reversible over 10 alternating cycles. When gadolinium was present at 10 μM, relaxation times shortened dramatically. When DTPA removed the gadolinium, times returned to baseline. This reversibility distinguishes NV-based sensing from conventional molecular probes that accumulate signal and cannot be reset.
Living cells presented additional complexity because nanodiamonds move considerably inside them, driven by intracellular transport and whole-cell migration. The team developed an automated tracking system that monitors fluorescence intensity and triggers repositioning scans when signal drops below a threshold. One tracked particle traveled 15.04 μm over 140 minutes while undergoing continuous T₁ measurement. Such trajectory data adds a new dimension: movement patterns reveal information about cytoplasmic viscosity and cellular activity states.
Organelle-specific measurements required surface modifications. For mitochondrial targeting, the researchers attached a mitochondria-targeting sequence rich in positively charged and hydrophobic amino acids. This modification shifted surface charge from −12.86 mV to 25.37 mV. Colocalization measurements showed targeting efficiency increased from 0.16 for bare particles to 0.60 for functionalized ones.
Mitochondrial sensors responded clearly to metabolic stress. Researchers added CCCP, a drug that uncouples oxidative phosphorylation and increases reactive oxygen species production. This disruption increases reactive oxygen species production. After CCCP addition, the T₁ relaxation time of mitochondria-targeted nanodiamonds decreased significantly. Control experiments with identical drug concentration in cell-free environments showed no T₁ change, confirming the response arose from biological rather than chemical effects.
Nuclear targeting proved more challenging. The researchers combined a cell-penetrating peptide called TAT with a nuclear localization signal to help particles cross both the cell membrane and nuclear envelope. Three-dimensional confocal imaging and transmission electron microscopy confirmed successful nuclear entry, though efficiency remained low because the 40 nm particle size approaches the transport limit of nuclear pores.
Comparing relaxation times between nucleus and cytoplasm yielded an unexpected finding. Across 43 particles in each compartment from 11 HeLa cells, no significant difference emerged. Nuclear T₁ averaged 110.65 ± 14.74 μs versus 114.34 ± 12.53 μs in cytoplasm. Both values were substantially shorter than T₁ measured in complete culture medium (228.22 ± 43.15 μs), reflecting elevated concentrations of paramagnetic species inside cells compared to their surroundings.
Limitations persist. Individual nanodiamonds show inherent variability in their relaxation properties even under identical conditions, complicating quantitative comparisons. Nuclear targeting efficiency needs improvement. Smaller nanodiamonds closer to 5 nm would be ideal for precise localization, but most particles that small lack the crystal structure required for quantum sensing.
This work establishes a framework for tracking metabolism in living cells with subcellular resolution over extended periods. By combining stable, reversible quantum sensors with organelle targeting and particle tracking, the approach opens possibilities for studying how different cellular compartments coordinate metabolic activity. Understanding such coordination, and how it fails in diseases like cancer and neurodegeneration, demands exactly this kind of spatially and temporally resolved measurement.
