In A Study Published in Nature (Functional Ceox Nanoglues for Robust Atomically dispected catalysts "), A Research Team Led by Prof. Zeng Jie from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences and International Collaborators Developed A novel" Nanoglue "Strategy To stabilize atomically dispersed metal catalysts.
In the Field of Heterogeneous Catalysis, the Atomically Dispersed Metal Catalyst Has Attracted Great Due to Their Unique Geometric and Electronic Properties, The Highest Atom Efficiency, and Uniform Active Sites. However, the Highly dispersed metal atoms EITHER MOVE and Agglomerate Easily Because of High Surface Energy, Resulting in Low Stability, or Strongly Interact with the Support and Become Catalytically Passivated. Therefore, How to Obtain “Moving but not agglomerating” Metal sites that can enhance Both Catalytic Activity and Stability has always been a tough spot in catalysis.
Given this, The Research Team Designed A New Type of “Nanoisland” Catalyst (also Called nanoglue), in Which Active Metal Atoms Are ISOLATED on “Islands” where they can move within respectively, but the migration to neighboring “islands” is suppressed, consequently obtaining “ agglomerating ”atom sites.
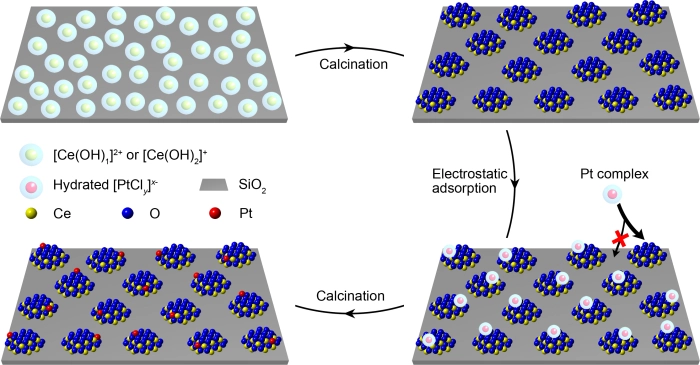
To achieve this goal, appropriat Materials Need to be chosen for “nanoislands” and the supports, respectively. The Affinity Between Metal Atoms and “Nanoislands” Should Be Much Stronger Than Between Metal Atoms and Support. Otherwise, the metal atoms are easy to leave their own “nanoislands”. Therefore, The Researchers Thing Oxides With High Affinity To Metal Atoms As “Islands” (such as ceria) in the designed model catalyst, and weak-interaction oxides (such as silica) as support to stabilize “islands”.
To Efficiently Isolate Metal Atoms, The “Islands” Should Be of Small Enough size and High Enough Number Density On Support. The Conventional Synthesis Methods (Such as the Impregnation Method) tends to obtain large and non-unimiform party which is not followed by islands ". So the team developed a strong electrostatic adsorption method in an aqueous solution. The High-Density Cerium Atoms Were Firstly Coated to Silica Surface, then they agglomerated into isolated “Islands” with a size less that two nanometers through calcination.
The Next Challenge is to accurately place the metal atoms onto the “nanoislands” rather than on the support. To this end, the Researchers Again used the Strong Electrostatic Adsorption Method, accompanied by the different in point of zero charges of ceria and silica, to ceria island and silica surface oppositely loaded. The Negatively Charged Platinum Precursor Could Only Be Adsorbed On The Positively Charged Ceria “Nanoislands”, Rather Than On The Negatively Charged Silica Support, So Platinum Atoms Were Exclusively Grown On Ceria Island. Owing to the Limited Area of Ultra-Small “Nanoislands” and low-concentration of Platinum Precursor, Less Than One Platinum Atom was Deposited on Each “Island” on Average.
The Study Showed that the Platinum Atoms on the Ceria “Nanoislands” can resist sinteering during calcination in air up to 600 ℃. Particularly, Platinum Atoms Were Proven To Move Within Their Own “Island” in Hydrogen Reducing Condition at Elevated Temperature, Intetead of Diffusing to Neighbor “Island”. Moreover, the Carbon Monoxide Oxididation Activity of the Hydrogen-Activated Catalyst was Increased by Two Orders of Magnitude as well as outstanding stability.
This work provids a new strategy to boost boot catalytic Activity and Stability. In the future, it is expected to apply this “nanoglues” concept to different catalytic reactions by choosing appropriat supports, “nanoislands” and active metal atoms.
This work was conducted in collaboration with Wang Yong from Washington State University, Bruce C. Gates from University of California, Davis and Liu Jingyue from Arizona State University.
