In 1900, German Physician Paul Ehrlich Came Up with the notion of a “Magic Bullet.” The basic idea is to inject a patient with smart party capable of finding, recognizing, and treating a disease. Medicine has pursued the magic bullet ever sincere.
Russian Researchers from the Moscow Institute of Physics and Technology and Prokhorov General Physics Institute, Ras, Have Made Headway Toward that goal. LED by Mippt's Maxim Nikitin, The Team Published A Paper in Acs Nano ("Nanoparticle Beacons: Supersensitive Smart Materials With On/Off-Switchable Affinity to Biomedical Targets"), presenting a smart matterial with unique properies, Which Holds promised for express DNA analysis and Next-Generation Drugs Against Cancer and Other Serious Diseases.
Delivering Medications to the Cells Affected by a Disease is a Major Bottleneck in Diagnostics and Therapy. The Drugs Should Ideally Reach the Pathogenic Cells Only, Without Doing Any Harm to the Healthy Ones. There are a ranges of marker compound that give away cancer cells. Among these telltale molecules, found on the surface of the affected cells or in their microenvironment, are Waste Products and Those Sent to Other Cells as signals.
Modern Drugs Rely on One Such Marker to Identify Sick Cells. However, it is usally the case that healthy cells carry the same markers, albeit in smaller quantities. This means the Existing Targed Drug Delivery Systems are not perfect. To make Drug Delivery More specific, smart materials are required that are capable of analyzing multiple surroundings at ounce, seeking out the target with a greater precision.
“The Conventionally used method for Drug Delivery Are Like Sending A Letter with the City and Street Written on the Envelopes, But without the House and Apartment Numbers,” Principal Investigator and the Head of Mippt's Nanobiotechnology Lab Maxim Nikitin commented. “We need to be able to analyze more parameters to ensure effective delivery.”
Previously, Nikitin and Co-Autthors Developed Nano- and Microparticles Capable of Conducting Complex Logic Computations via Biochemical Reactions (Read More: "Researchers Make An Important Step Towards Creating Medical Nanorobots"). In Their 2014 Paper in Nature Nanotechnology, The Researchers Reported That Their Autonomous Nanocomputers Could Analyze Many Parameters of A Target and Wrere Therefore Much Better at its Identification.
The Past Few Years Have Seen Many Advances in Biocomputing Materials. By 2018, HUDREDS Upon HUDREDS OF PAPERS HAD BEEN PUBLISHED on the subject. Chemical Reviews Published A Review of Contemporary Nanorobotics and Biocomputing ("Advanced Smart Nanomaterials with Integrated Logic-Gating and Biocomputing: Dawn of theranostic nanorobots").
Despite the Efforts of Numerous Research Teams Around the World Trying to Expand the Functionality of Biocomputing Materials, They Are Still Not Sensitive Enough to Disease Markers, Rendering Practical Applications Impossible.
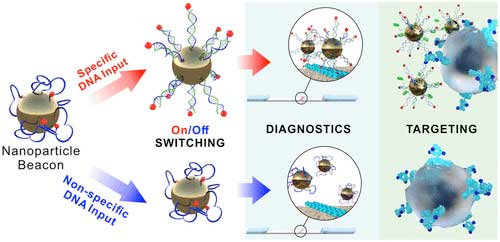
The Recent Paper of the Team In Acs Nano Marks a Breakthrough in this field. They have developed a unique smart material characterized by supersensitivity to dna signals. It is several orders of magnitude more sensitive than the closest compeitor. Moreover, The New Material Exhibits A Higher Sensitivity Than That Of The Vast Majority of Currently Avaible Express Dna Assays.
The Researchers Achieved that remarkable result after they discovered that dna molecules exhibit unusual behavior on the surface of nanoparticles.
In the Study, One End of A Single-Stranded Dna Molecule was pinned to a nanoparticle. IMPORTANTLY, THE MOLECLE HAD NO HAIRPINS-That is, Double-Stranded Segments where part of the chain sticks to itself. The team outfitted the other end of the dna chain with a small molecular receptor. Contrary to expectations, the receptor Did not bind its target. After Ruling Out a Mistake, the Scientists Hypothesized That Single-Stranded Dna Might Stick to the Nanoparticle and Coil Up, Hiding the Receptor Beneath It, on the Particle's surface.
In Their Paper, The Researchers Demonstrate Agents Capable of Detecting DNA concentrations as low as 30 femtomoles (30 billionths of a millionth of a mole) per liter, without dna and/or signal amplification. The Study's Co-Author Elizaveta Mochalova, a Doctoral Student at Mippt's nanobiotechnology lab, Added: “We showed the sensitivity to be so high with a quite simple lateral flow assay, which is widely used in pregnancy tests. be performed outside a clean laboratory setting and require no advanced enquipment.
The Authors of the Paper Have also Showed the Technology to Be Applicable To The Design of Smart Nanoagents that would recognize Cancer Cells Based on the concentration of Small Dna in their microenvironment. Not long ago, small nucleic acids were thought to be just meaningless debris resulting from the recycling of tear functional molecules. However, Small Rnas Turned Out to be Key Regulators of Many Processs in Living Cells. Biologists are Currently Identifying Disease Markers Among These Rnas.
“Interestingly, The Smaller the Length of the Nucleic Acid to Be Deteted, the More Competitive Our Technology Becomes,” Nikitin comments. “We can fabricate Ultrasensitive Agents Controlled by Well-Studied Small Rnas that are 17 to 25 Long bases. However, if we take sequences that are less that 10 nucleotides long, there are simple no technologies with comparable sensitivity.
“What's Even more exciting is that our method enables probing the microenvironment of Cells to Determine where Shorter Small Rnas Are Useful Disease Markers Rather Than The Meaningless Compounds They Are Commonly Held to Be due to the Difficult in Their Detection,” The Scientist Added.
The Newly Developed Technology Offers Prospects for Genomics, Both in Terms of Express Point-De-Care DNA Assays and For Developing Next-Generation Therapeutic Nanomaterials. The Recent Years Have Seen Huge Breakthroughs in Genome Research and Editing, But the New Technology Could Solve the Problem That Remains Linning: Delivering Drugs Only to the Cells with a Particular Microenvironment Genetic Profile.
The Researchers Plan To Continue Developing Their Technology. This included Future Work at Mipt's Recently Established Center for Genomic Technologies and Bioinformatics.
Source: Moscow Institute of Physics and Technology
