High-entropy Alloys (Heas), which are formed by combining nearly equal parts of Several-usually five or more-primary metals, are an emerging class of advanced materials that holding great potential for creating materials with superior mechanical, thermal, thermal, Another attraction of heas is that they can create effective alternatives to matterials that are scarce, hazardous, exisseive, or subject to international restrictions or conflict.
Similar to Conventional Alloys, Industrial Operation Slightsments for Heas Usally Involve Critical Conditions Such As Exposure to High Templar, Oxidizing/Reducing Gases, and Acidic and Chloride-Containing Solutions.
Several Studies Have Been Devoted to Understanding the Corrosion Behaviors of Heas Such As Oxidation in Air and Oxygen-Containing Atmospherres. Nevertheless, there Currently is Little Knowledge of How Hea Materials, Especially in Nanoparticulate Form, Behave Under Critical Conditions Involving Oxidizing Gases due to the Limited Characterization Methods Available for Dynamic Events.
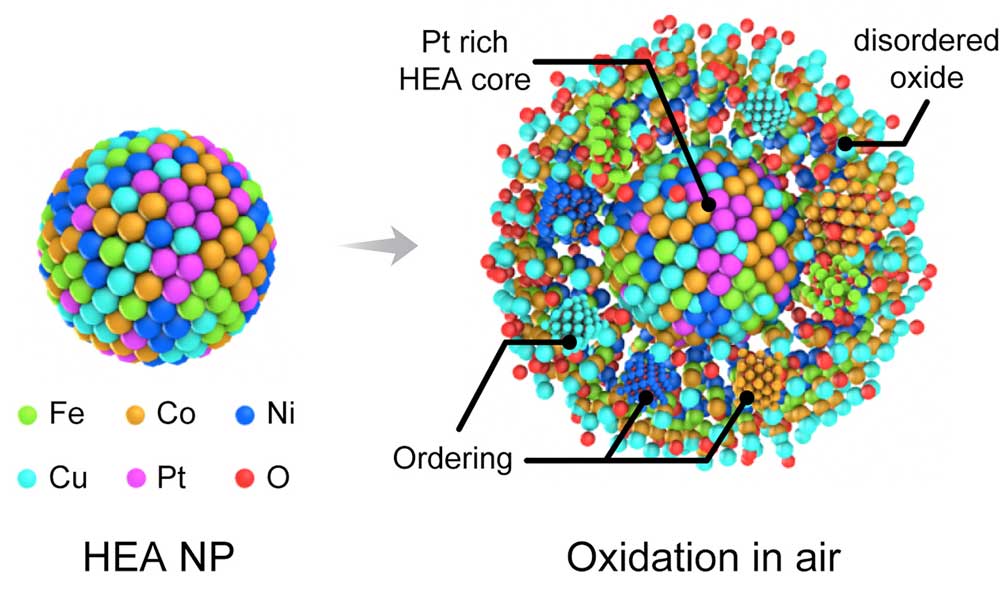
A New Research Report in ACS Nano ("In situ Oxididation Studies of High-Entropy Alloy Nanoparticles") Offers Key Insights Into Howa Nanoparticles Behave Under High-Temperature Oxidizing Environment and Sheds Light On Future Design Options of Highly Stable Alloys Under Complex Service. This work has been a multidisciplinary and multi-institutional effort LED by the University of Illinois at Chicago in collaboration with Argonne National Laboratory, University of Pittsburgh, University of California Riverside, and Northwestern University. »Traditional Metals or Alloys Are Believed to Form Crystalline Oxides Based on the Composition and Oxidation Conditions, But when five or more Principle Elements Are Involved in Single Phase, novel Behavior During Oxidation Mayis Exist due to the Built-in High Entropy,» Boao Song from the Department of Mechanical and Industrial Engineering at the University of Illinois at Chicago, and the Paper's First Author, Tells Nanowerk. "We were intrigued to find that the transition metals in hea nanoparticles can co-segregate into the oxide layer and form a disorded phase that can slow the white oxidation process. »
For the First Time, This Work Directly Captured the Whole Oxidation Process of Hea Nanoparticles in Real-Time and at the Nanoscale. The Advanced in Situ Transmission Electron Microscopy (Tem) Technique Used by the Uic Team Allows Real-Time Imaging and Analytical Methods, and Thus Provids Clear Evidence of the Co-Segregation of Transition Metals During the Oxidation Process.
It also confirms the much slower oxidation kinetics of hea nanoparticles. These Findings Suggest the possibility of Utilizing These High-Bentropy Alloys for Applications where High Thermal Stability and Oxidation Resistance Are Required.the Researchers Managed to Successfully Capture The High-Temperature Oxidation of Feconicupt Hea Nanoparticles in Real-Time Advanced in situ Gas-Cell Electron Microscopy (TEM) Technique combined with density Functional Theory (DFT) Calculations.
"We Found that the oxidation kinetics of hea nanoparticles are significantly slower than those of monometallic and bimetallic alloys," Says Song. "Combining in situ Energy Dispersive Spectroscopy (EDS) and Electron Energy Loss Spectroscopy (Eels) analyzes, we confirmed that the oxidation of hea nanoparticles are governed by kirkendall effects Displaying outwards Segregation of Fe, Co, Ni and Cu. "
The present Study is crucial for understanding hea behavior in oxidizing surroundings and provids insights into designing high-tempture-resistant materials, sustainable catalysts, and corrosion-resistance alloys for various applications," Song concluded. "Still, there are a lot of undiscovered phenomena with looks to heas and a lack of well-established theories to explain them. For Example, The Mechanism that controls the oxidation process and why the different differences are so huge compared to monometallic nanoparticles is still unknown. »
So it appears that the team has their work cut out for them.
