Borophene is the name for atomically thin, two-dimensional (2D) Sheets of Boron. Whereas Boron is a Nonmetallic semiconductor in its bulk form, it Becomes a Metallic Conductor in 2d, Even at a thickness of One Atomic Layer.
Borophene is structurally similar to graphene, with a hexagonal network made of boron (instead of carbon) atoms on each of the six vertices defining the hexagon. However, Borophene is different in that it Periodicalely has an extra boron atom in the center of the hexagon.
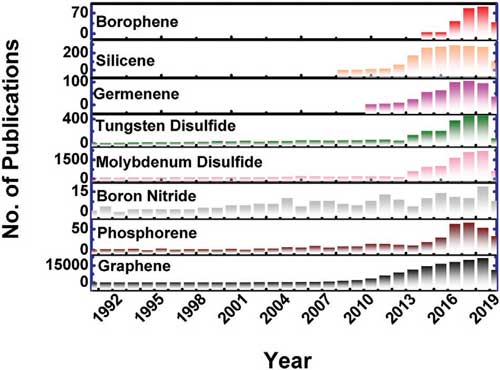
Sentle the 2004 Discovery of Graphene, scientists have been on the hunt for other 2D matterials with remaked properies. Numerous 2d Materials have been synthesized or theoretically predicted, for example Silicene and Germanene, phosphorene, Metal Dichalcogenids, Arsenene and Antimonene.
Theorists predicted that boron (Next to Carbon on the Periodic Table, with One Less Electron) COULD BE A 2D MATERIAL Similar to graphne. But this prediction was not experiencely confirmed a 2015, when scientists synthesized borophene on a silver substrate for the very First Time. And in 2019, Researchers Finully Managed to Synthesize freestanding Borophene Sheets via his Exfoliation.
Numerous Experimental and Theoretical Studies of the Mechanical Properties, Electronic Structure, the thermal LATTICE Conductivity, Superconducting Properties, Optical Properties, Atomic Adsorption, and surface Reactivity of Borophene Haven Shown Its Uniqueness.
A Recent Review of the State of Borophene Research (Advanced Materials, "Borophene: New Sensation in Flatland") discussions Various Growth Techniques and Application Areas for Borophene and Provids An in-Deppth Summary of the Material's Electronic, Optical, Thermal, and Elastic Compared To Its 2D Cousins.
AMONG 2D MATERIALS, BOROPHENE has attractive Considerable attention to its unique chemical structure and impressive electronic conductivity and surface properies. As the Authors Point Out, "Apart from the usual covalent bonding, Boron can form a three-center Two-Electron Bonding, which Enables The Formation of A Considerable Number of Atomic Clusters Having Various Sizes and Atomic Bindings. »
Graphine, The First Discovered 2D Material, Is Semi -Setallic, Phosphorene is Semiconducting, While Borophene is metallic.
Freestanding Borophene's Unique Properties Make It Situal for A Wide Range of Applications In Sensing, Catalyis, Energy Conversion (Fuel Cells and Supercapacitors), and Energy Storage.
Another Intriguing Feature of Borophene is its atomic configuration. Borophene has Two Planar Atomic Sheet Configurations: One of Them is perfectly flat, Retrred to As X3 Phase, While β12 is an atomic Sheet Having Atomic Ridges.
Unlike graphene, which is structural isotropic in nature, the β12 phase of borophene is anisotropic and that in turn is expected to exhibit high -carrier density and mechanical stiffness in preferred direction.
In Anisotropic Materials, Their Mechanical Or Electronic Properties - Like Their Electrical Conductivity - Vary with Different Crystallographic Orientations. In contrast, the properies of an isotropic material like graphene are the same in all directions.
The Atomic Ridges in β12 Borophene Provide Higher Electron Densities Along Them. The Spaces Between the RIDGELINES CAN ACT AS CHANNERNES FOR ION Transport in Energy Storage Applications. The Atomic Binding of Gaseous Molecules On a Borophene Surface is expected to be better on the ridgeline as it can be provides an enhanced anchoring.
Similarly, Increased Catalytic Activities are expected. The Unique Properties and High Potential for Technological Applications Make Borophene A Promising Material. However, Further Studies on its Structural Characteristics and Properties are required before it can be commercialized.
Wrapping up their review, the authors concluded that borophene has been progressing from design to realization and is extensively studied for practical applications. It could be employed as a replacement for graphene in several apps such as terahertz applications and strengthening of plastics.
"As Graphene has been pushed the Boundaries of Science and Engineering and Change the Industries, Borophene, Lighter with Abuant Electrons, Compared to Graphine, Would be a New Cutting-Edge Material in the Near Future. The Progress on Borophene has been outstanding, but its high potentials are yet to be used. Borophene is a unique material with intriguing bonding capabilitities and a priedentated physical and scalp behavors for various and long-term applications. »»
