The ability to convert carbon dioxide into useful chemicals and fuels could help address rising atmospheric CO2 levels while simultaneously producing valuable industrial feedstocks. The electrochemical reduction of CO2 to carbon monoxide is particularly attractive, as CO serves as a key building block for producing various chemicals through established industrial processes like Fischer-Tropsch synthesis. However, achieving high efficiency and selectivity in CO2 electroreduction has remained challenging due to several fundamental obstacles. The chemical stability of CO2 molecules makes them difficult to transform, while competing reactions – particularly the production of hydrogen – often dominate instead of the desired CO formation.
« For practical applications of CO2 reduction catalysts, achieving high selectivity across a wide potential range is essential but remains a significant challenge, » Professor Jin Kon Kim at Pohang University of Science and Technology (POSTECH) tells Nanowerk.
Scientists have explored various catalytic materials to overcome these challenges, with single metal atoms coordinated to nitrogen atoms on carbon supports (M-Nx/C) showing particular promise. These single-atom catalysts maximize the utilization of metal atoms while providing high selectivity for CO production. However, conventional methods for preparing these catalysts have made it difficult to understand exactly how they work.
« Most single-atom catalysts are synthesized through high-temperature pyrolysis, which inevitably alters the coordination environment of the active sites, » explains Kim. « This makes it difficult to analyze how support properties affect performance in isolation. »
Kim’s team tackled this problem by taking a fundamentally different approach. Instead of using high-temperature processing that generates mixed coordination environments, they first created carbon support materials with precisely controlled properties, then attached well-defined nickel-containing molecules to provide uniform catalytic sites. This systematic approach allowed them to directly study how the support material’s structure and composition influence catalyst performance.
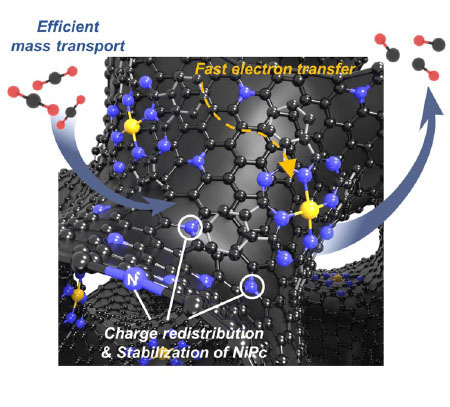
The researchers discovered that both the physical structure and electrical properties of the carbon support play crucial but distinct roles. The degree of graphitization – which affects electrical conductivity – proved especially important for achieving high CO selectivity at lower voltages. However, the ability to effectively transport CO2 molecules through the material emerged as an even more critical factor for overall catalyst activity.
By optimizing these properties, the team created a catalyst that maintains over 90% selectivity for CO production across a wide range of applied voltages – a significant improvement over previous materials. The best-performing catalyst combined moderately high graphitization with an ordered mesoporous structure (pores 2-50 nanometers in diameter) and nitrogen doping.
Detailed characterization revealed why this combination works so well. The mesoporous structure allows CO2 molecules to readily access the active sites throughout the material. The graphitic character provides good electrical conductivity to supply electrons for the reduction reaction. Meanwhile, the nitrogen atoms incorporated into the carbon framework help anchor the nickel catalyst molecules in place while also tuning their electronic properties to favor CO2 reduction over competing hydrogen evolution.
Looking ahead, Kim identifies key challenges that remain: « In the field of single-atom catalysts for CO2 reduction, simultaneously optimizing the properties of active sites and supports remains significant. While the support can enhance mass transport properties and improve overall catalytic performance, the intrinsic catalytic activity is fundamentally determined by the active site properties. »
The insights gained from this systematic study should help guide future efforts to develop even more effective catalysts. The ability to maintain high selectivity across a broad voltage range is particularly important for potential industrial applications. The clear design principles established for the roles of porosity, conductivity, and chemical composition provide a strong foundation for further advances in converting CO2 into useful chemical products.
