Gels occupy unusual territory among materials. They behave like solids but consist mostly of liquid, holding their shape under gravity yet yielding when squeezed. A gel traps solvent within a matrix while still permitting dissolved molecules to diffuse through. Some gels can even repair themselves after damage and flow through narrow openings before resolidifying, making them attractive for injectable drug delivery.
Scientists have exploited these properties in applications ranging from wound dressings to controlled therapeutic release. But conventional gels offer limited selectivity. They can slow diffusion, yet they struggle to distinguish between molecules of different sizes or charges. Achieving precise control over which molecules a gel captures, and how quickly it releases them, has remained an elusive goal.
Nanosheet-based gels promised greater discrimination. Clays and graphene oxide, both composed of thin flat sheets, stack and interlock to form three-dimensional networks that immobilize solvents effectively. Researchers have deployed them in water treatment, energy storage, and biomedical devices.
Yet these materials share a fundamental constraint: the sheets themselves contain no internal pores. Molecules interact only with surfaces and the gaps between sheets, not through any channels that might filter based on molecular dimensions. Selectivity depends entirely on surface chemistry and the tortuous paths between stacked layers.
Metal-organic frameworks presented a possible solution. These crystalline materials, built from metal ions connected by organic linker molecules, contain precisely sized pores capable of capturing, storing, or separating molecules with high selectivity. Scientists have pursued framework-based gels by fusing growing crystals during synthesis or by combining crystals with polymers. But loading cargo into such gels proves slow and often uneven. The dense three-dimensional structure that provides selectivity also resists rapid penetration. Soaking a preformed gel in a cargo solution can take days to achieve adequate loading, and the distribution often remains patchy.
Two-dimensional relatives of these frameworks, called metal-organic nanosheets, offered an alternative path forward. These ultrathin sheets retain the ordered internal porosity of their three-dimensional cousins while presenting vastly more accessible surface area. Researchers have incorporated them into membranes for gas separation and into sensors for trace chemical detection, exploiting their ability to discriminate molecules by size. Whether these nanosheets could form gels, and what unique capabilities such gels might possess, no one had systematically investigated.
A study published in Advanced Functional Materials (« Metal‐Organic Nanosheet Gels: Hierarchically Porous Materials for Selective Loading and Differential Release ») now fills this gap. Researchers at Nanjing University of Posts and Telecommunications and the University of Sheffield demonstrate that metal-organic nanosheets form gels through simple centrifugation. The resulting materials possess hierarchical porosity, combining micropores within each nanosheet with larger voids between them. This dual-scale architecture enables selective loading and differential release of molecules based on both size and electrical charge, with release timescales spanning from 6 hours to approximately two weeks depending on molecular properties.
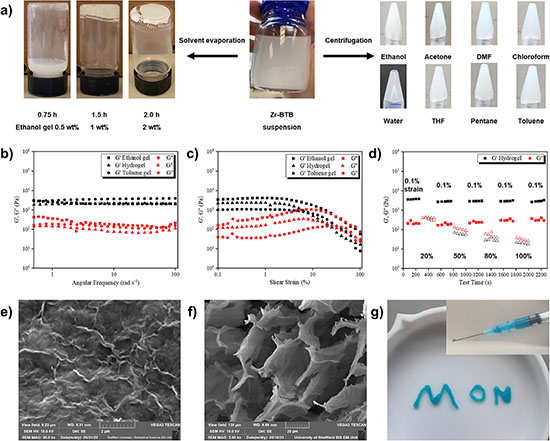
Synthesis followed established methods. Heating zirconium chloride and an organic linker called 1,3,5-benzenetribenzoate in a mixture of formic acid, water, and dimethylformamide at 120 °C for 24 hours produced a suspension of ultrathin sheets. Atomic force microscopy confirmed single-layer structures roughly 1.5 nm thick, and gas adsorption measurements yielded a surface area of 360 m² g⁻¹.
Gel formation emerged unexpectedly. While attempting to isolate nanosheets from ethanol by centrifugation, the researchers noticed that about one-fifth of the solvent had become immobilized at the bottom of the tube. When inverted, the material held its shape—a simple but widely accepted indicator of gelation.
A reliable protocol followed: dispersing 25 mg of nanosheets in 10 mL of ethanol and centrifuging at 4500 rpm for one hour produced approximately 2 mL of gel containing about 1.5 wt% nanosheets. Gelation occurred across eight solvents, including acetone, chloroform, toluene, and water.
Rheological testing confirmed true gel behavior. At low strain, the storage modulus (measuring solid-like elasticity) exceeded the loss modulus (measuring liquid-like flow) by roughly 10-fold across a wide frequency range. Hydrogel samples recovered mechanical properties within seconds after disruption, demonstrating the rapid self-healing behavior that makes gels suitable for injection through fine needles. This resilience allowed the material to be extruded into complex shapes that held their form for hours.
Zeta potential measurements revealed that dilute nanosheet suspensions carry an overall positive surface charge of 30.6 mV. At high concentrations, favorable van der Waals attractions appear to overcome electrostatic repulsion between sheets, causing them to overlap and stack in misaligned arrangements that trap solvent while forming a continuous network.
Freeze-drying hydrogel samples produced self-supporting aerogels. Electron microscopy showed overlapping nanosheets forming a honeycomb-like network with voids tens of micrometers across. Combined with intrinsic micropores within each sheet, this structure creates two distinct length scales of porosity.
Centrifugation proved especially valuable for loading cargo. Forming the gel in the presence of dissolved cargo molecules distributed them uniformly throughout the material in just one hour. Conventional soaking required approximately 72 hours to achieve comparable loading and often produced uneven distribution.
Five model compounds served as test cargoes. Two neutral molecules, trans-anethole and carbamazepine, showed minimal uptake because they interacted weakly with the nanosheets. Charged molecules loaded far more efficiently: methylene blue reached 65.6%, methyl orange achieved 77.2%, and brilliant blue G attained 68.5%. Electrostatic attraction between the positively charged nanosheets and these charged species explains the difference.
Release experiments revealed the gels’ most distinctive behavior. Fresh ethanol layered atop cargo-loaded gels allowed tracking of diffusion using UV-visible spectroscopy.
Trans-anethole, the smallest neutral molecule tested, reached equilibrium concentration in about 6 hours. Carbamazepine, a larger neutral molecule, required roughly 18 hours. A « fish and fishing net » analogy explains this difference. The nanosheets contain pores approximately 0.54 nm wide. Trans-anethole molecules measure about 0.43 nm across—small enough to slip through these openings—allowing them to take direct paths out of the gel. Carbamazepine molecules span 0.67 nm, too large to fit through the pores. They must navigate around the sheets, following longer and more tortuous routes.
This pattern inverts expectations from conventional porous materials. In traditional gel-permeation chromatography, smaller molecules become trapped in pores and exit more slowly. Here, two-dimensional geometry creates the opposite effect: small molecules escape faster because more pathways lie open to them.
Charged molecules exhibited markedly slower release. Methylene blue, carrying a positive charge, required about 34 hours to reach equilibrium. Negatively charged methyl orange and brilliant blue G took approximately two weeks. Positively charged nanosheet surfaces bind these anionic molecules through electrostatic attraction, and sulfate groups on the dyes may coordinate reversibly with zirconium ions, further extending residence time.
Drug delivery systems might exploit such differential release to administer multiple therapeutics at programmed rates from a single injectable formulation. Water purification devices could capture contaminants selectively based on size and charge. Sensing platforms might benefit from the high surface area and self-healing behavior.
Because metal-organic frameworks are highly tunable, future researchers could engineer nanosheets with customized pore sizes and surface chemistry, adjusting release profiles for specific applications. By combining the processability of nanosheet gels with the molecular discrimination of porous frameworks, this research establishes a new materials platform for capture, separation, and controlled release.
