A team of researchers at KAIST has unveiled a breakthrough technique that could transform clean energy production. Using a flash of light lasting just 0.02 seconds, the method generates temperatures of 3,000°C to rapidly create high-performance catalysts with dramatically lower energy use.
The findings are published in ACS Nano (« Photothermal Annealing-Enabled Millisecond Synthesis of Carbon Nanoonions and Simultaneous Single-Atom Functionalization »).
This ultrafast process consumes over a thousand times less energy than traditional techniques and boosts hydrogen production efficiency by up to six times. The discovery marks a major stride toward making hydrogen energy more viable for large-scale use.
A joint team, led by Professor Il-Doo Kim from the Department of Materials Science and Engineering and Professor Sung-Yool Choi from the School of Electrical Engineering, developed what they call a “direct-contact photothermal annealing” platform. The technique synthesizes advanced nanomaterials by exposing them to a brief but intense burst of light that produces extreme heat almost instantly.
Using this light energy, the researchers converted chemically stable nanodiamond precursors into conductive and catalytically active carbon nanoonions (CNOs). Even more striking, the method simultaneously attaches single metal atoms to the surface of these CNOs, combining two complex steps into one rapid process.
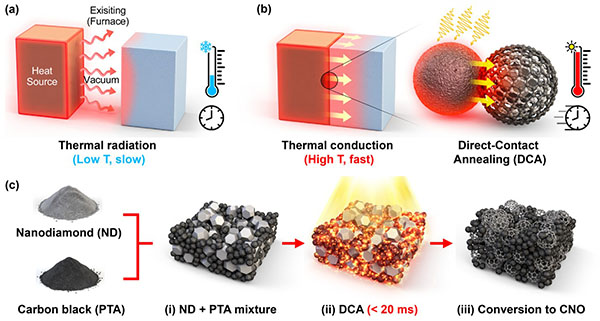
CNOs, made of concentric layers of carbon, are known for their excellent conductivity and chemical stability, making them ideal supports for catalysts. But conventional production methods have required multiple heating and post-processing stages that consume vast amounts of energy and are difficult to scale up.
To overcome those challenges, the KAIST team mixed nanodiamonds with light-absorbing carbon black and exposed the mixture to a xenon lamp pulse. The light instantly transformed the nanodiamonds into CNOs—a reaction confirmed by molecular dynamics simulations.
When the researchers added metal precursors such as platinum, cobalt, or nickel, the metals broke down during the flash and attached as single atoms to the surface of the newly formed CNOs. The rapid cooling prevented the atoms from clumping together, producing a uniform structure in one seamless step.
So far, the team has successfully synthesized eight varieties of single-atom catalysts. Among them, platinum-loaded CNOs showed a sixfold improvement in hydrogen evolution efficiency compared to conventional catalysts, while using far less precious metal.
“We have developed, for the first time, a direct-contact photothermal annealing process that reaches 3,000°C in under 0.02 seconds,” said Professor Il-Doo Kim. “This ultrafast synthesis and single-atom functionalization platform reduces energy consumption by more than a thousandfold compared to traditional methods. We expect it to accelerate the commercialization of technologies in hydrogen energy, gas sensing, and environmental catalysis.”
The discovery could help make clean hydrogen production both faster and more sustainable, offering a powerful tool for the next generation of green technologies.
